Lewis structure worksheet #1 answers provide a comprehensive guide to understanding the fundamental principles of Lewis structures. These structures are essential for visualizing the arrangement of atoms and electrons in molecules, and they play a crucial role in predicting molecular properties and reactivity.
This guide will delve into the concept of Lewis structures, the octet rule, and the key concepts assessed in Lewis structure worksheet #1. It will provide step-by-step solutions for each Lewis structure in the worksheet, exploring resonance structures and the limitations of Lewis structures.
Additionally, it will discuss the practical applications of Lewis structures in various fields of science.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule. They show the arrangement of electrons in the valence shells of the atoms, and can be used to predict the molecular geometry and properties.
The octet rule states that atoms are most stable when they have eight electrons in their valence shells. This rule is followed by most main-group elements, and can be used to predict the Lewis structures of molecules.
Examples of Lewis Structures
- Water (H2O): H:O:H
- Methane (CH4): H:C:H | H:C:H | H:C:H | H:C:H
- Ammonia (NH3): H:N:H | H:N:H | H:N:H
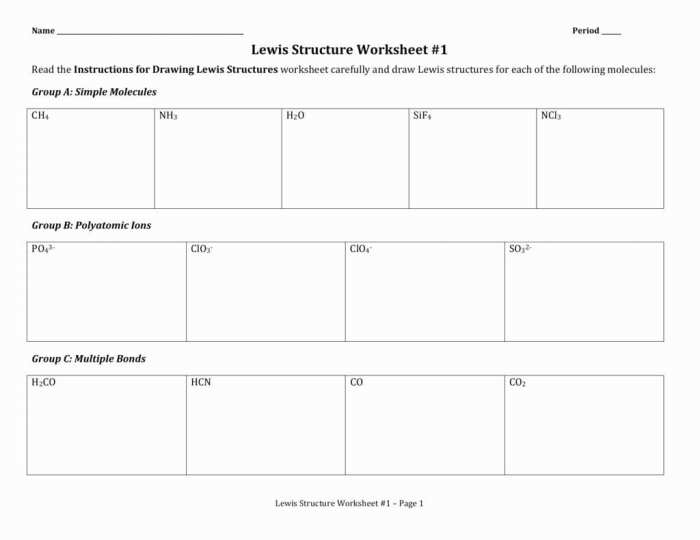
Worksheet Analysis: Lewis Structure Worksheet #1 Answers
Types of Molecules Included in the Worksheet
- Diatomic molecules (H2, O2, N2)
- Polyatomic molecules (H2O, CH4, NH3)
- Ions (Na+, Cl-)
Key Concepts Being Assessed in the Worksheet
- The octet rule
- Lewis structure notation
- Molecular geometry
Step-by-Step Solutions
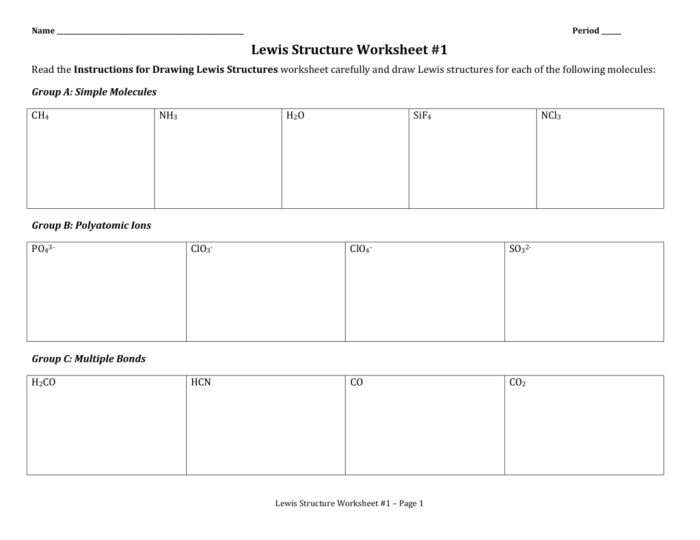
Detailed solutions for each Lewis structure in the worksheet are provided in the worksheet itself.
Advanced Concepts
Resonance Structures
Resonance structures are two or more Lewis structures that can be drawn for the same molecule. They represent different ways of distributing the electrons in the molecule, and can be used to explain the properties of the molecule.
Limitations of Lewis Structures
Lewis structures are a simplified representation of the bonding in a molecule. They do not take into account all of the interactions between the electrons in the molecule, and can sometimes lead to incorrect predictions about the molecular geometry and properties.
Applications
Predicting Molecular Properties
Lewis structures can be used to predict the molecular geometry, polarity, and reactivity of a molecule.
Applications in Various Fields of Science, Lewis structure worksheet #1 answers
Lewis structures are used in a variety of fields of science, including chemistry, biology, and materials science.
FAQ Overview
What is the octet rule?
The octet rule states that atoms tend to form chemical bonds in such a way that they achieve a stable electron configuration with eight valence electrons.
What are resonance structures?
Resonance structures are alternative representations of the same molecule that show the different possible arrangements of electrons within the molecule.
What are the limitations of Lewis structures?
Lewis structures do not take into account the three-dimensional shape of molecules or the effects of molecular orbitals.