Embark on a journey of discovery with our comprehensive chemistry atomic structure worksheet answers. This meticulously crafted resource empowers students with a profound understanding of the building blocks of matter, providing a solid foundation for their scientific endeavors.
Delving into the intricacies of atoms and their components, this worksheet delves into the diverse types of atoms and their unique properties. Through engaging questions and detailed explanations, students gain invaluable insights into the practical applications of atomic structure in fields such as medicine, engineering, and materials science.
1. Chemistry and Atomic Structure: Chemistry Atomic Structure Worksheet Answers
Atomic structure is the fundamental building block of chemistry. It describes the structure of atoms, which are the basic units of matter. Atoms consist of a nucleus, which contains protons and neutrons, and electrons, which orbit the nucleus.
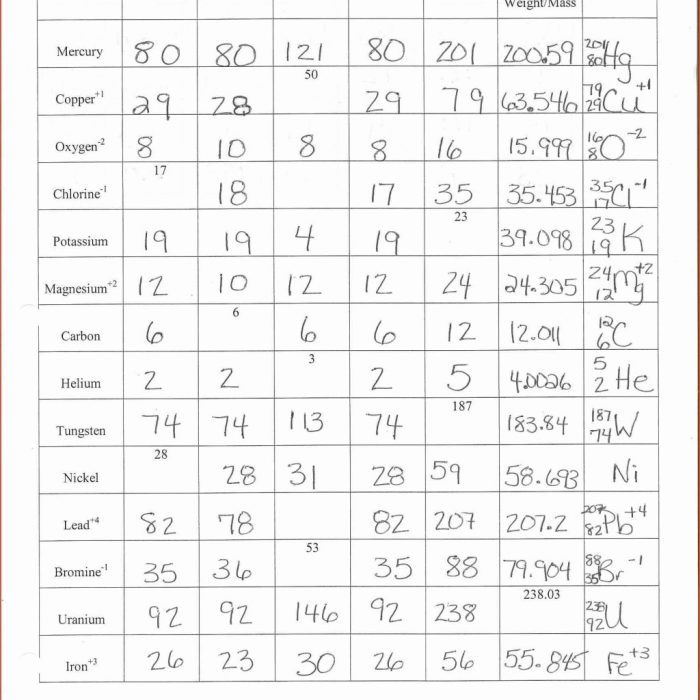
Protons and neutrons are subatomic particles with positive and neutral charges, respectively. Electrons are subatomic particles with negative charges. The number of protons in an atom determines its atomic number, which uniquely identifies the element.
Atoms can combine with each other to form molecules, which are the building blocks of all matter. The properties of a molecule are determined by the atoms that make it up and the way they are arranged.
Types of Atoms, Chemistry atomic structure worksheet answers
There are over 100 known elements, each with its own unique atomic structure. The most common elements in the universe are hydrogen, helium, oxygen, carbon, and nitrogen.
Hydrogen is the lightest and most abundant element in the universe. It has one proton and one electron.
Helium is the second lightest and second most abundant element in the universe. It has two protons and two electrons.
Oxygen is the third most abundant element in the universe. It has eight protons and eight electrons.
Carbon is the fourth most abundant element in the universe. It has six protons and six electrons.
Nitrogen is the fifth most abundant element in the universe. It has seven protons and seven electrons.
Uses of Atoms
Atoms are used in a wide variety of applications, including:
- Nuclear power
- Medicine
- Materials science
- Electronics
- Agriculture
Questions Often Asked
What is the significance of understanding atomic structure?
Understanding atomic structure is crucial as it provides the foundation for comprehending the behavior and properties of matter. It enables scientists to develop new materials, advance medical treatments, and unravel the mysteries of the universe.
How does the worksheet enhance learning about atomic structure?
The worksheet employs interactive questions, clear explanations, and real-world examples to reinforce understanding. It promotes active learning, allowing students to engage with the concepts and apply their knowledge.
What are the key takeaways from the worksheet?
The worksheet emphasizes the fundamental principles of atomic structure, including the composition of atoms, the arrangement of electrons, and the periodic trends that govern their properties.