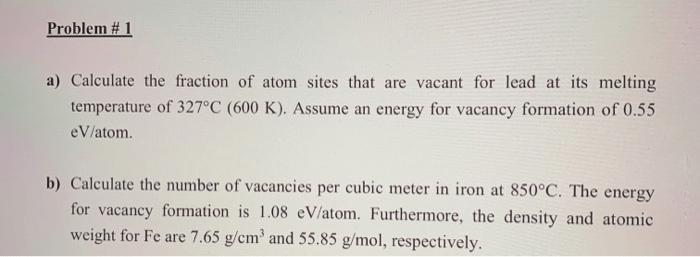
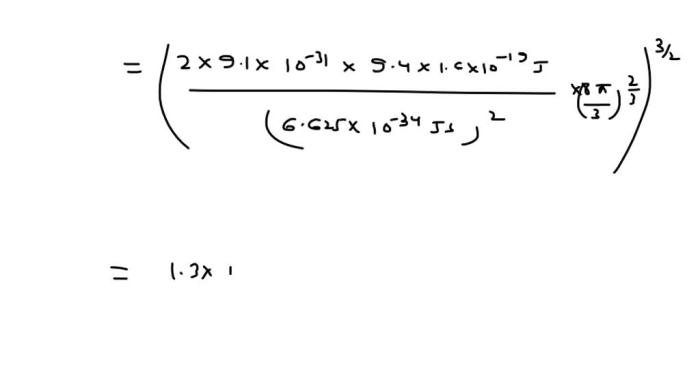Calculate the fraction of atom sites that are vacant – Vacancy sites, where atoms are conspicuously absent, play a pivotal role in shaping the properties of materials. Understanding the fraction of vacant atom sites is paramount in materials science and engineering, enabling us to tailor materials for specific applications and unravel the intricacies of atomic-level phenomena.
This comprehensive guide delves into the methods for calculating vacant atom site fraction, exploring the Monte Carlo method, statistical mechanics, and experimental techniques. We will elucidate the factors influencing vacancy formation, including temperature, impurities, and crystal structure. Finally, we will showcase the applications of vacancy site fraction calculations in materials science, highlighting their impact on material properties and the exciting field of vacancy engineering.
2. Methods for Calculating Vacant Atom Site Fraction

Monte Carlo Method, Calculate the fraction of atom sites that are vacant
The Monte Carlo method is a computational technique that simulates the behavior of a system by randomly sampling possible states and calculating the average outcome. In the context of vacancy site fraction calculations, the Monte Carlo method can be used to simulate the creation and annihilation of vacancies in a crystal lattice.
The simulation starts with an initial configuration of the lattice, where each site is either occupied by an atom or vacant. The system is then allowed to evolve by randomly selecting a site and attempting to create or annihilate a vacancy at that site.
The probability of success of these attempts is determined by the temperature and other factors that influence vacancy formation.
The simulation is run for a large number of steps, and the fraction of vacant sites is calculated as the average number of vacant sites over the course of the simulation.
Statistical Mechanics
Statistical mechanics provides a theoretical framework for understanding the behavior of systems with a large number of particles, such as atoms in a crystal lattice. In the context of vacancy site fraction calculations, statistical mechanics can be used to derive equations that describe the equilibrium concentration of vacancies in a system.
These equations take into account the temperature, the energy required to create a vacancy, and the entropy of the system. By solving these equations, it is possible to calculate the fraction of vacant sites in a system at equilibrium.
Experimental Techniques
Experimental techniques, such as X-ray diffraction, can be used to estimate the vacancy concentration in a material. X-ray diffraction is a technique that uses X-rays to probe the structure of a material. The intensity of the X-rays diffracted from a material is sensitive to the presence of defects, such as vacancies.
By analyzing the intensity of the diffracted X-rays, it is possible to determine the concentration of vacancies in a material. This technique is often used to characterize the vacancy concentration in materials that are used in semiconductor devices and other applications where the presence of vacancies can significantly affect the material’s properties.
Popular Questions: Calculate The Fraction Of Atom Sites That Are Vacant
What is the significance of calculating the fraction of vacant atom sites?
Calculating the fraction of vacant atom sites provides valuable insights into the atomic-level structure and behavior of materials. It helps us understand the formation and annihilation of vacancies, the influence of impurities and defects, and the impact of crystal structure and bonding on vacancy concentration.
How can the fraction of vacant atom sites be used to tailor material properties?
Vacancy engineering, based on controlling the fraction of vacant atom sites, is a powerful tool for tailoring material properties. By manipulating vacancy concentrations, we can influence diffusion, electrical conductivity, mechanical strength, and other important material characteristics.